About Us
As an institution, INNOVACORE Center for Research & Biotechnology is registered with the Office of Human Research Protection, US Department of Health & Human Services
Institutional Organization & Registration #: IORG0012140
INNOVACORE Center for Research & Biotechnology has also filed a Federal Wide Assurance with the Office of Human Research Protection, US Department of Health & Human Services
Federal Wide Assurance #: FWA00034338
The Office of Human Research Protection Program (OHRPP) manages all clinical research conducted under the guidance of the INNOVACORE Center for Research & Biotechnology. OHRPP in partnership with the research community is responsible for ensuring the safety and welfare of participants in clinical trials conducted under the aegis of INNOVACORE
OHRPP also provides administrative support to the INNOVACORE Institutional Review Board (IRB). It is also involved in facilitating the submission of clinical protocols by physician-investigators in various Centers of Excellence in INNOVACORE
INNOVACORE Center for Research & Development currently follows the AAHRPP Accreditation Standards. Accreditation from the Association for the Accreditation of Human Research Protection Program (AAHRPP) demonstrates the excellence of a research program which provides the most comprehensive protections for research participants
INNOVOCORE IRB is registered with the Office of Human Research Protection, US Department of Health & Human Services
OHRP IRB #: IRB00014365
CITI Training Program
INNOVACORE Center for Research & Biotechnology has partnered with the CITI (Collaborative Institutional Training Initiative) to offer training modules specifically designed for researchers and other professionals involved in research. Key components of CITI Training program for research involving human subjects are depicted below:
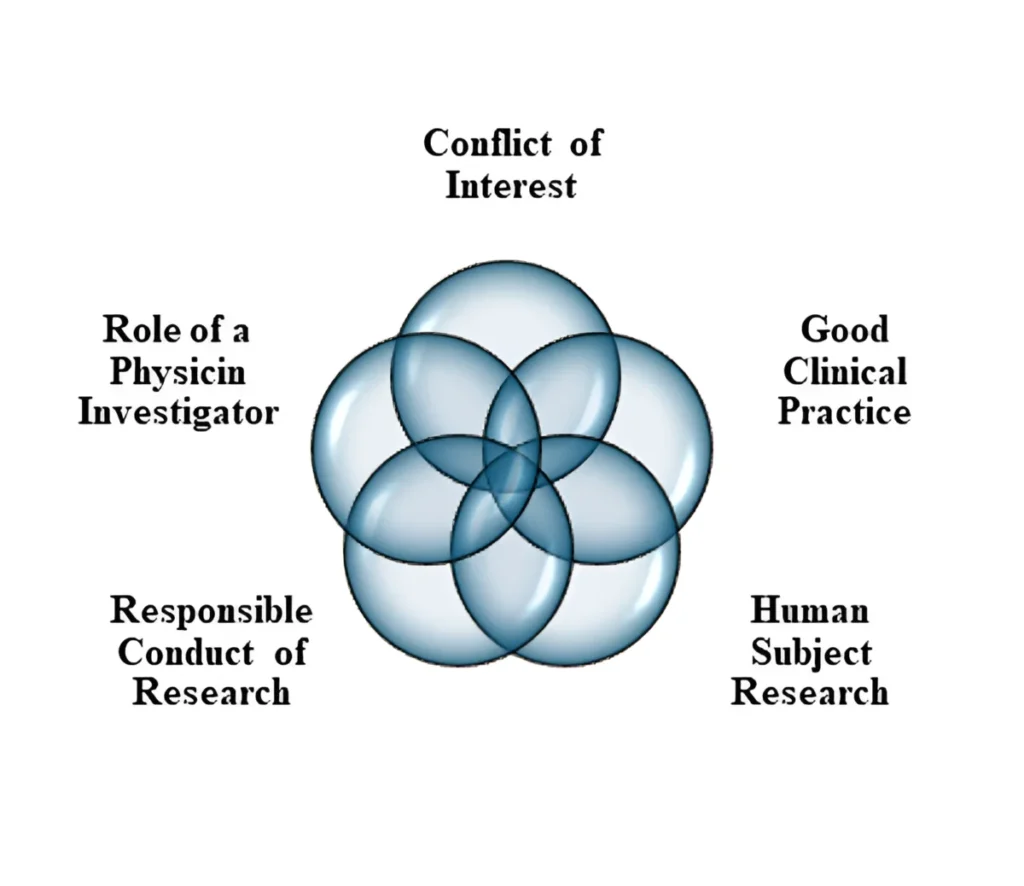
To learn more about CITI training
